
Pismo zakonodavcu: Cjepivo protiv COVID-19 moglo bi nas razboliti više od COVID-19
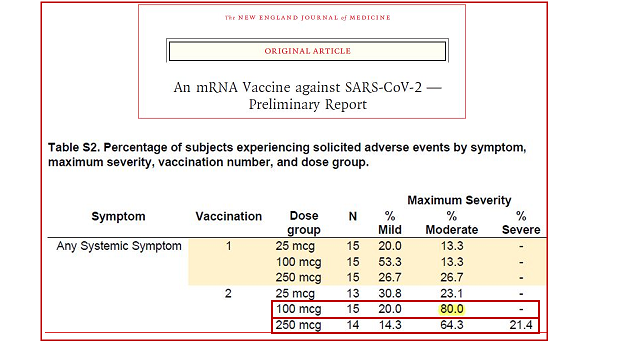
Dana 14. srpnja 2020. godine stručni medicinski časopis New England Journal of Medicine (u daljnjem tekstu NEJM) objavio je preliminarno izvješće o rezultatima ispitivanja 1 faze “Moderninog” cjepiva protiv COVID-19 pod nazivom: An mRNA Vaccine against SARS-CoV-2 – Preliminary Report. Unatoč iznimno visokom postotku umjerenih do teških posljedica na zdravlje nakon primljene druge doze, zaključak znanstvene skupine bio je da “nisu utvrđeni ograničavajući sigurnosni problemi u ispitivanju. Ovi rezultati podržavaju daljnji razvoj ovog cjepiva “
Obzirom na šokantan zaključak znanstvenika koji ispituju sigurnost cjepiva protiv COVID-19, cjepiva koje se pokazalo da izaziva visoki postotak umjerenih do teških posljedica na zdravlje ljudi na kojima je vršeno ispitivanje, tvrde da nema sigurnosnih problema za daljnji razvoj cjepiva, grupa “West Virginian for Health Freedom” je poslala svim zastupnicima u saveznoj američkoj državi West Virginia slijedeće pismo:
Poštovani zakonodavče,
- Rezultati studije cjepiva Moderna Faza 1 protiv COVID-19 objavljeni su u utorak u NEJM-u.
- 80% zdravih ispitanika, u prosjeku starosti 33 godine, cijepljenih s dozama od 100 mcg i 250 mcg doživjelo umjerene do teške posljedice po zdravlje nakon 2. cijepljenja.
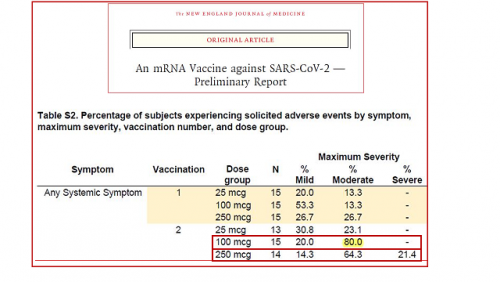
- Umjerene do teške posljedice po zdravlje (engl. Moderate severity reaction) izazvane cjepivom protiv COVID-19 definirane su kao jedno ili više sljedećih stanja koji ometaju ljudsku aktivnost: artralgija (bol u zglobovima), umor, groznica pri zemperaturi od 38,5 ° C, zimica, mijalgija (bolovi u mišićima), opetovana upotreba lijekova protiv bolova duže od 24 sata ili više od 5 centimetara crvenila ili oteklina na mjestu ubrizgavanja.
- Oni koji se zaraze virusom SARS-CoV-2 koji izaziva bolest COVID-19, njih od 40 do 80% nema nikakvih simptome bolesti.
- Iz toga proizlazi da će više ljudi oboljeti od cjepiva nego COVID-19.
- Ljudi koji se zaraze COVID-19, njih 40 – 80% nemaju nikakve simptome bolesti.
- Budući da je Moderna izbjegla testiranje na životinjama, dok se cijepljene osobe ne izlože SARS-CoV-2 virusu nećemo znati hoće li se kod njih razviti ozbiljne, potencijalno fatalne ozljede pluća, kao što se dogodilo u prethodnim istraživanjima SARS cjepiva provedenim na životinjama.
Izvor: https://childrenshealthdefense.org/news/vaccines/letter-to-wv-legislators-the-moderna-covid-19-vaccine-is-likely-to-make-more-people-sick-than-covid-19/
Zdravnik dr. James Neuenschwander , specialist urgentne in integrativne medicine je strokovnjak za motnje imunskega sistema. On je pojasnil, kaj je patologija povezana s cepivi za respiratorne virus, kot je SARS CoV-2 in se imenuje "antibody immune enhancement": Če cepimo 300 milijonov američanov in bo 1% imelo škodljive posledice, bomo imeli 3 milijone novih bolnikov z avtoimunskimi boleznimi. Več o tem je v komentarjih 6--7-8-9 na koncu objave.
Dr. Sherri Tenpenny: zdravilo klorokin je učinkovito in zato cepivo za COVID-19 ni potrebno https://freedomplatform.tv/dr-sherri-tenpenny-face-masks-are-not-effective-against-covid-19-how-masks-are-being-used-to-control-the-population/?fbclid=IwAR0I0gkPGx-HHg6-lwtW_yCavayvPaMA0MEWTxEGGVu9lIFEpLa59YnughM
Covid 19 cepivo v Rusiji https://www.nytimes.com/2020/08/02/world/europe/russia-trials-vaccine-October.html?fbclid=IwAR21HZQRDCVkV5HkX81KJLDI8DaTnPxJJPWuMQJLByA3l6t8mAaHfA-lI4c
https://pubmed.ncbi.nlm.nih.gov/22536382/
https://www.youtube.com/watch?v=Dlr0_YFJDz4&feature=emb_logo
https://cen.acs.org/pharmaceuticals/vaccines/Adenoviral-vectors-new-COVID-19/98/i19
https://cen.acs.org/pharmaceuticals/vaccines/Four-COVID-19-vaccine-candidates/98/i29
* * *
WHO je finančno odvisna od donacij zasebnega kapitala povezanega z izdelovalci cepiv.
V Sloveniji je bila 3. 9. 2020 v Zakonu o nalezljivih boleznih bolezen Covid19 uvrščena v prvo skupino nalezljivih bolezni, kjer so kuga, hemoragična mrzlica in druge izjemno nevarne bolezni. Bojim se, da se s tem utira pot za sprejem zakona o obvezni množični COVID vakcinaciji državljanov.
EU je 300 milijonov odmerkov cepiva že plačala. Z argumentom kugi podobno nevarna bolezen, je zakonodaja proizvajalcem Covid cepiv že pred tem dovolila registracijo za množično vakcinacijo državljanov brez predhodnega ugotavljanja morebitnih dolgoročnih škodljivih učinkov cepiva na ljudi.
dr. Vladimir Pirnat
*
The company published details of the trials on Saturday, after facing criticism over the lack of transparency surrounding the testing of the much-anticipated vaccine against the virus, which has so far infected more than 30.8 million people and caused over 958,000 fatalities worldwide.
The first participant of the British trials – which are being conducted in conjunction with Oxford University – fell ill after receiving one dose of the experimental vaccine in July.
The female volunteer was later diagnosed with transverse myelitis, a rare inflammatory disease that affects the spinal cord, causing weakness, sensory alterations, and autonomic nervous-system dysfunction. The company’s spokeswoman later told the media the volunteer had undiagnosed multiple sclerosis, and the trials resumed.
* * *
Walter Veith, zoolog o cepivu https://www.youtube.com/watch?v=Qk5_lmxUzF8&feature=youtu.be
https://www.lifesitenews.com/news/trials-of-uk-covid-vaccine-derived-from-aborted-baby-cell-line-paused-after-participant-falls-ill?utm_source=LifeSiteNews.com&utm_campaign=be94a63f7a-Freedom_9_10_2020&utm_medium=email&utm_term=0_12387f0e3e-be94a63f7a-407977330
*
Will covid-19 vaccines save lives? Current trials aren’t designed to tell us
Dear Editor
A newly published article by Timothy Cardozo and Ronald Veazey “Informed consent disclosure to vaccine trial subjects of risk of COVID‐19 vaccines worsening clinical disease” reports on a study to determine whether or not sufficient literature exists “to require clinicians to disclose the specific risk that COVID-19 vaccines could worsen disease upon exposure to challenge or circulating virus.” (1)
The authors concluded that it did.
The results of the study………….
“……..that vaccines designed empirically using the traditional approach (consisting of the unmodified or minimally modified coronavirus viral spike to elicit neutralizing antibodies), be they composed of protein, viral vector, DNA or RNA and irrespective of delivery method, may worsen COVID-19 disease via antibody-dependent enhancement (ADE). This risk is sufficiently obscured in clinical trial protocols and consent forms for ongoing COVID-19 vaccine trials that adequate patient comprehension of this risk is unlikely to occur, obviating truly informed consent by subjects in these trials.”
The authors concluded that………….
“The specific and significant COVID‐19 risk of ADE should have been and should be prominently and independently disclosed to research subjects currently in vaccine trials, as well as those being recruited for the trials and future patients after vaccine approval, in order to meet the medical ethics standard of patient comprehension for informed consent.”
Along with an inability to prove a reduction in the likelihood of severe illness and hospitalisation and prevent infection in interrupting transmission etc the current trials may also be falling foul of what constitutes informed consent from trial participants in failing to disclose a “specific and significant” Covid-19 risk of ADE.
Looking to the future, the authors concluded that the risk should also be disclosed to “future patients after vaccine approval”.
The fact that Covid-19 vaccines could worsen disease when exposed to challenge or circulating virus, is something that every individual should be aware of before consenting to vaccination.
*
By Julie Beal
There’s a lot more to this ronascam than we first thought – I did a little digging, to understand the new vaccines, and it became clear that part of the ‘reset’ the UN and WEF have announced is a future dominated by proteins and genetic hybrids.
There are genetically engineered proteins for vaccines, brewed in bioreactors inside cells from insects or bacteria, and there are bioreactors for ‘food’ products, etc., and a whole range of things, including pharmaceuticals. Proteins are also what the ronavax are all about, with some of them even using ‘fake DNA’ to turn our own bodies into bioreactors, churning out the proteins the genetic code instructs us to. Your body is a protein factory. Why pay for a bioreactor and wait months for them to ‘grow’? Injecting the code straight into us saves time and money.
- The new coronavirus (SARS-CoV2) vaccines are totally different to any vaccines that have been approved before, and yet they’ve been in development for over twenty years. There have been some genetic treatments made available, so why have no vaccines for humans been licensed?
- The investors want results, to bring the products to market, and now a global experiment is being proposed.
- The novel ingredients in the ronavax include DNA plasmids, modified mRNA, lipid nanoparticles, and the adenovirus envelope; but none of these have been licensed for humans before either!
- The vaccines are plug-and-play ‘therapeutics’, so they are using the same platform each time but with a different genetic insert. They are all much cheaper and quicker to produce than the standard vaccines.
- Patents for some of these platforms refer to the inclusion of xeno nucleic acid (XNAs), or unnatural amino acids (for instance, to produce mRNA) – these are synthetic analogues of ‘the real thing’. ‘Xeno’ means ‘not found in nature’ or ‘alien’.
- The ronavax may also contain novel molecular adjuvants – that’s because genetic vaccines (let’s call them ‘genvax’) are said to need a lot of adjuvants, to make you have a strong immune response (pah!) – so instead of just the usual toxins being added (such as alum), more genetic code is added, e.g. for cytokines – this is also a very new and worrying gimmick.
- The vaccines are part of the field of gene therapy which has managed to bring some treatments to market for serious illnesses such as cancer and diseases caused by missing or defective genes, but not so for the many vaccines, using the same platforms, which have also been designed and tested.
- All of the platforms have been tested for over twenty years – there’s been a lot of tweaking of methods with some apparent success, but no profit.
- There have been at least two deaths and a lot of illness and adverse events during trials, most of which are said to be under-reported. During the inquiry into Jesse Gelsinger’s death, for instance, “the FDA and NIH revealed that 691 volunteers in gene-therapy experiments had either died or fallen ill in the seven years before Jesse’s death; only 39 of these incidents had been reported promptly as required.”
- It’s acknowledged by the experts that there’s a chance of cancer, autoimmune diseases and other problems, but they don’t say what happened to people more than a year or two after clinical trials, because nobody seems to check!
- Millions of $ have been poured into genvax and other gene therapies, but still nothing has been brought to market – why?
- Perhaps the ronascam is partly about overcoming the problems with getting genvax to market quickly – the laws arising from the Convention on Biodiversity (CBD) have blocked many genetic patents from success over the years, but it seems to be giving up its power to the WHO! Bill Gates seems?? to have tried to influence the CBD about gene drives in 2017. The Nagoya Protocol and the Cartagena Protocol of the CBD are clearly standing in the way of a ‘free and open market’ for genetic products and patents, but the ronascam could enable the WHO to reframe Agenda 21 in favour of biotech.
- From now on, no matter what, the answer will be: “Because virus!”
- Ultimately, the ronavax could open the door to more and more genetic patents, involving ‘alien’ life forms, etc., which could lead to the loss of biodiversity.
- +

+
Crikey, this is going to take a lot of explaining … so keep taking a breather! I’ve written a series of articles, packed full of original sources, most of which are academic, peer-reviewed articles, and are freely available online. I’ll begin here with a short overview of my forthcoming articles about the connection between the ronascam, the vaccines, and the control of genetics.
- Ronavax Rundown – a quick guide to the ronavax and what is said to be in them.
- A Guide to the Ronavax – Understanding the Experimental Coronavirus Vaccines – more detail about the types of ronavax winning the race, who is making them, and how they work.
- Gates’ way to genetic control? Here comes the EA Swine Flu! The main selling point of genvax is their super-quick turnaround time – just give the vax makers the genetic sequence, and they can whip up a ‘cure’ in no time. And it could be claimed the vector is the same and has been trialled, so all that’s needed is to SHARE the data globally so they can design some new genetic code for a vaccine, and save the world from the new swine flu.
- Ronavax Roulette – Potential problems with the corona-virus vaccines – the potential ill effects of the vaccines, as suggested by the World Health Organization, and by geneticists themselves; a look at previous trials and what went wrong when using much the same platforms, including the deaths of Jesse Gelsinger and Ashanthi DeSilva, and the shocking consequences of the TeGenero incident; molecular adjuvants.
- WHO’s in Charge of Biodiversity? Biotech means messing with DNA in so many ways, but has been scuppered thus far by the Convention on Biodiversity (CBD), Cartagena and Nagoya Protocols. This includes patents involving genetics. Even countries who are not signatories are restricted by the Protocols when it involves a relationship with a country that is a signatory. The WHO is now claiming a link between health and the environment. And did Bill Gates try to influence the CBD about gene drives in 2017?
- Protein shame – the darkest side of biotechnology; the rise of so-called therapeutics and so-called food based on proteins. The future being planned for us involves food replacement (as detailed by Ice Age Farmer) from upcycled chicken and grains to proteins produced in bioreactors, such as Gates’ fake meat and breast milk. Some biotech industries rely on animal cruelty, questionable abortions, and the lust for young blood and tissue in the quest for regeneration.
- Ronavax – understanding synthetic biology and XNA – which scientists also call ‘alien DNA’ because it is ‘not of this world’ and the potential reprogramming of our physical reality. Plus a look at the use of XNA in gene therapy; for instance, the mRNA used in the ronavax is almost real, but not quite – it uses nucleotide analogues for parts of the code that’s created. There is also a push to use XNA as a ‘genetic firewall’ against viruses, as it is said this would be safer than using gentech based purely on natural DNA.
https://www.youtube.com/watch?time_continue=2&v=gG7uCskUOrA&feature=emb_logo
Julie Beal is a UK-based independent researcher who has been studying the globalist agenda for more than 20 years, focusing on a wide range of information around Agenda 21, Communitarianism, Ethics, Bioscience, and much more.
Note, first of all, that there are actually about 150 ronavax in development, but the likes of AstraZeneca, Pfizer, Sanofi, Novavax and Moderna are the ones with the contracts – they also have the clinical trials in place, and the ones using the most concerning techniques.
There are four main types of ronavax being developed, all of which are genetically engineered and loaded with toxins: the first type contain either bits of virus or bits of protein the virus makes (i.e. virus like particles and protein sub-units). This approach is similar to how traditional vaccines are said to work, in that they contain something the body is meant to interpret as a virus, to cause us to launch an immune response (e.g. by producing antibodies). The proteins for the vax are first manufactured in a vile broth in bioreactors by adding DNA to cells from various animals, insects, bacteria, etc. However, it takes months for the proteins to ‘grow’ or replicate using this method.
The other three kinds (the ones making the news and winning the contracts) are the genetic vaccines (let’s call them genvax). They all use methods of getting DNA or mRNA into the cells of the body using a ‘vector’, which is a delivery vehicle to protect the genes as they travel from the site of injection towards cells in the blood, tissues, and organs. Each of these vectors encodes the protein for the alleged rona, which means it contains the instructions for the body to make the protein itself.
These three vectors are:
- a genetically modified virus, e.g. a monkey cold virus (simian adenovirus)
- lipid nanoparticles containing mRNA
- bacterial plasmids (a ring of DNA found in bacteria)
The vectors are used in gene therapy, and they have been in development for 30 years! But none have been licensed! Some of them contain not just DNA, but also XNA or xeno nucleic acids (where ‘xeno’ means alien, or not found in nature). The idea is to expand the genetic code.
Plus, as I show in other articles about the ronavax, the vaccine rollout could also lead to widespread use of genetic patents, by using the World Health Organization to over-rule the Convention on Biodiversity, and thus unleash monstrous synthetic, ‘alien’ life forms and health interventions, and so much more.
An important thing to note about the vector vaccines is that they are plug-and-play platforms. The vector remains the same, only the code is different. This means that once the technology is rolling, pumping out vaccines on a daily basis, they can easily make a slightly different code and pop that into the vector instead. Within days, they could be pumping out a vaccine for a new virus, rather than the rona.
So perhaps instead of, or as well as, a ‘second wave’, there will be another type of virus which either does or does not cause a real pandemic. Then it could be claimed that the genvax are the only ones that can be made in time to ‘save’ us.
Other concerns are that the vax will contain RFID chips, hydrogel, or microneedles, or use a quantum dot tattoo, making us trackable, and controllable. Whilst all of these technologies exist, none of them are planned, allegedly, to be used in the ronavax, except for one vaccine that uses microneedles (see below).
Hydrogel
Of all the uses for hydrogel listed on Wikipedia (other than contact lenses, diapers, and hydrogel pads for burns and blisters), the uses that might relate to vaccines and/or biosurveillance include:
- Environmentally sensitive hydrogels (also known as ‘Smart Gels’ or ‘Intelligent Gels’)
- Injectable hydrogels which can be used as drug carriers for treatment of diseases
- Sustained-release drug delivery systems
- Hydrogels that are responsive to specific molecules,[11] such as glucose or antigens, can be used as biosensors
Hydrogel can be combined with lipid nanoparticles, so perhaps some of the vaccines that use lipid nanoparticles (e.g. Moderna) use hydrogel.
Microneedle array
The Pittsburgh Coronavirus Vaccine (PittCoVacc) is delivered through “a fingertip-sized patch of 400 tiny needles that deliver the spike protein pieces into the skin, where the immune reaction is strongest. The patch goes on like a Band-Aid and then the needles — which are made entirely of sugar and the protein pieces — simply dissolve into the skin.”
Quantum dots and luciferase
Luciferase is a bioluminescent gene that can be synthesized and used for a variety of purposes. For example, a firefly gene which encodes luciferase can be incorporated into other synthetic DNA then injected into the body – it is sometimes used in trials of DNA vaccines, so that the way the DNA spreads throughout the body can be monitored, because it GLOWS!
Luciferase can even be injected into a plant with a gene gun as a gene editing technique to make the plant glow, as described in this video.
Biocompatible, near-infrared quantum dots have been proposed for vaccines where they can be ‘read’ by a smartphone to check the person has received the ‘right’ vaccine, at the required time. It’s been proposed that microparticles containing quantum dots, and embedded in dissolvable microneedles, be used.
Gene editing
It’s entirely possible that this is the plan – but to produce a particular effect on the genome, the DNA being injected would have to be designed to target specific parts of the body, for instance by using CAS-9. The International Commission on the Clinical Use of Human Germline Genome Editing has just been set up (September, 2020) to provide guidance on using gene editing to make changes that are passed on to children (called ‘heritable genome editing’). Gene editing in a child or adult can create changes that affect the individual but they aren’t passed on to offspring (that we know of).
Microchips
This is something many people have raised concerns about for decades now, and has never seemed so likely as in this age of government terrorism. So yes, perhaps the vaccines will contain something zombifying, or sterilizing, or a microchip. But perhaps it’s more likely we’ll be forced to use a smartphone and/or have a biometric/microchipped ID. At least for now. This has been accelerated by the global coercion campaign to use a ‘health app’ to rejoin society.
Why pay to put chips in the vax when many people won’t survive the coming hardships anyway? Even the poorest people in the world have had to pay for being given a global ID number. And they weren’t given a microchip – their fingerprints were catalogued instead.
As part of the push to enrol millions in the ID2020 program around the world, hikers, skiers and pack animals carrying equipment were used to reach poor people in less developed countries, so they could be signed up to the ID scheme. These people had to pay (around $1) to give up their biometrics for their ID, even though they would probably never use it, given the lack of power and internet connectivity. And, to stop them stealing the equipment, “registration officers were the first to be issued IDs, which were then linked to a specific kit”.
In much the same way, people living in industrialized countries will probably pay for their own enslavement; whether it’s a chip in the phone, or under the skin, it’ll be up to the individual to go out and get it. The complexities of digital identification these days are so multi-layered, they can’t all be put on a remotely-readable bio-chip. So, for now, microchips under the skin will probably just be linked to the phone with the apps required for leaving the house. Everywhere you go, you’ll need to show the green code for the health app on your phone to the police and to gatekeepers of shops, banks, etc., and it won’t be long until people get sick of doing this, and pay for a chip in their hand that’s linked to their phone, their ID, their money.
https://www.activistpost.com/2020/09/ronavax-rundown.html
A Guide to the Ronavax — Understanding the Experimental Coronavirus Vaccines
Around 150 vaccines are being developed for the alleged* rona virus, but the ones that deserve the most attention are the ones winning contracts and/or being trialled already, because their success is almost guaranteed. Some people want them, some never think about it, and others worry they could be coerced or physically forced to get them. The thing is, everyone should have access to information so they can make informed choices about what is right.
Two of these ronavax are vaguely similar to the kind of vaccines that have been licensed in the past. One is made by Sanofi/GSK – selected for Operation Warp Speed in the U.S., this vaccine contains proteins that are made in a bioreactor, using genetically engineered baculoviruses and insect cells. This is a method of protein engineering. Novavax, also funded by the US government uses a similar technique, but packages the proteins in nanoparticles. The idea behind these vax is the same as ‘normal’ vaccines, because the proteins are designed to look like the ones the rona is said to make (usually referred to as the spike or ‘S’ protein) so that your body reacts to them as if they were ‘the real thing’.
All the others are genetic vaccines, or ‘genvax’. They are very experimental, because they gain access to your cells and get you to be the bioreactor and make the proteins yourself.
This is done by smuggling genetic instructions into your cells, using one of three methods:
- Adenoviruses which have been genetically modified to contain or ‘express’ the gene of interest (used by AstraZeneca and J&J/Janssen)
- Lipid nanoparticles with mRNA (used by Pfizer/BioNTech, Curevac, and Moderna)
- DNA plasmids (used by INOVIO, a company worth noting because of its novel techniques, strategic partnerships and product pipeline)
They are all ‘delivery vehicles’ or ‘vectors’ – a way to get the DNA/mRNA into your cells.
They are all methods used in gene therapy. If the ronavax are licensed, not only could gene therapy become very big business, but restrictions on genetic patents could be loosened. Most current gene therapy products are for very specific conditions, e.g. LUXTURNA which uses the RPE65 gene for patients with an inherited retinal disease. However, because genvax use the same techniques, getting the ronavax to market could begin a new era of busy production lines in bio-factories, meaning they could also churn out some of the other ‘therapeutics’ that have been planned.
DNA and mRNA are said to be ‘the software of life’ and the possibilities are endless. So, perhaps, are the dangers. Because, whilst the platform or vector remain the same, the software doesn’t. For each new application, or disease, a new genetic code is designed on computers. Then, the new code is added to the chosen vector, whether it’s synthetic adenoviruses, bacterial plasmids, or nanoparticles. And each new code is a new risk. So, perhaps, is combining the codes – having more than one genetic vaccine, or drug, could create unforeseen effects.
Some of these platforms have patents which describe the use of XNA (xeno nucleic acids), as well as the lab-made DNA/mRNA – and all sorts of other things you might not realise!
Since they are all genetic vaccines, let’s call them ‘genvax’. They are very hard to understand when nobody has explained anything, but this series of articles will give information on some of the basics so you can wrap your head around it all!
[*The rona virus can only be ‘alleged’ because no causal link between what the rona tests are detecting, and any illness deemed to be ‘covid’, has been established. All we have to go on is ‘the news’, anecdotal reports, guesses, and a genetic sequence of ‘the rona’ provided by Zhang and Holmes by January 10th, 2020, and the abomination of ‘involving covid’ in the statistics. Be sure, at least, to check the mortality figures provided by your government and compare them to previous years! Most of the people who died in the beginning were already ill and old, and the lockdown meant their level of care was substantially reduced.]
The top seven ronavax, in terms of contracts awarded, are described below. Bear in mind that all of these vaccines:
- Are genetically modified or engineered (and some of them also contain genetic instructions for the body).
- Have been in development for a range of diseases for over 30 years.
- Received funding and support from the big boys.
- Can be tested and produced at enormous scale, according to the companies that make them (although hardly anyone is trained to do this because it’s never been done before).
- Have NEVER been licensed.
Only a handful of DNA vaccines have ever been licensed but they are all for veterinary use (e.g. for pigs and chickens).
The Frontrunners
AstraZeneca
- AZD1222; replication-deficient chimpanzee adenovirus vector; developed with Oxford University, and originally called ChAdOx1 nCoV-19; this vector has been used in trials for others vaccines, e.g. RSV vax; uses fetal cells in production; to be sold at cost; agreements with CEPI, GAVI and the Serum Institute of India to manufacture the vax.
- Funded by BARDA and NIH.
- About 60% of 1,000 participants in the phase 1/2 trial experienced side effects, and the study was put on hold because someone developed transverse myelitis, which is an inflammation of the spinal cord. It was later said to be an “unrelated neurological illness”.
- Phase 3 trials were also put on hold due to an adverse event for a UK participant. An informed consent form for this trial stated that effects would probably be mild to moderate, but could be severe, and that “you might experience pain resulting in some difficulty moving your arm, but this should resolve within a few days.” Other concerns were also stated, such as the possibility of enhanced disease and Guillain-Barré syndrome.
- More trials planned in America and Russia.
- A trial of ChAdOx1 85A prime with MVA85A boost (for tuberculosis) reported unsolicited adverse events, which included lymphopaenia, neutropaenia leukopaenia, eosinophilia and thrombocytopaenia, as determined by blood tests. One participant got shingles a month after the trial, but this was considered unrelated.
- Robert F. Kennedy Jr. reported: “Lead developer Andrew Pollard juggles scandalous conflicts that allow him to license, register, and mandate his own untested vaccines to the masses. Pollard is Senior Advisor to Britain’s MRHA Panel which licenses vaccines, chairs Britain’s JVCI committee that mandates them, and advises the European Medicine Agency (EMA). He takes payments from virtually all the big vaccine makers.”
- Struck a deal with Ethris in 2017 to use their proprietary mRNA technology for respiratory diseases.
- AstraZeneca may also be using, or planning to use, XNA in some of their products, e.g. modified mRNA and anti-sense oligonucleotides, as well as modified proteins, peptides and recombinant enzymes.
- Dose capacity 2.94 billion
Novavax
- NVX-CoV2373; recombinant nanoparticle vax using Matrix M™ adjuvant; $1.6 billion in funding from Operation Warp Speed plus funding from Dept of Defense, HHS and CEPI; doses for US citizens supplied at no cost; research trial with mice for MERS-COV vax in 2014 with funding from the NIH; flu vax called NanoFlu being trialled (the next step is to file for approval!) but gave up on their Ebola glycoprotein vax after phase 1; trials for their RSV vax called ResVax were also unsuccessful.
- In 2013, Novavax filed a patent application for a vax using an “immunogenic composition comprising a MERS-CoV nanoparticle”.
- Dose capacity 1.35 billion
Pfizer/BioNTech
- BNT162b2; using BioNTech’s nucleoside-modified messenger RNA (modRNA) platform which encodes an optimized SARS-CoV-2 receptor binding domain (RBD) antigen, and Acuitas Therapeutics’ lipid nanoparticles; several constructs being tested.
- In the phase I/II trial, one of the twelve participants who received the 100 µg dose (the highest dose) experienced severe pain at the injection site, and this group did not receive a second dose as planned.
- A fifth construct was announced at the start of September, and they all “use different mRNA formats and target antigens. Like its latest addition, dubbed BNT162b3, the two fast-tracked candidates use a nucleoside-modified RNA, or modRNA, while another prospect uses a uridine-containing mRNA, or uRNA, and the fourth uses self-amplifying mRNA.”
- Phase III trials are to include people with HIV and Hepatitis.
- Large manufacturing capacity.
- Early attempts used naked RNA, and were aimed at cancer.
- Founded in 2008, BioNTech’s partners have included Pfizer, Sanofi, Roche, Eli Lilly and Bayer.
- Product pipeline: 19 cancer vax, one for flu, one for covid, one undisclosed, others to follow, e.g. a HIV vaccine with the Bill and Melinda Gates Foundation.
- Dose capacity 1.3 billion
Sanofi/GSK (with TranslateBio)
- Adjuvanted recombinant protein-based vaccine.
- This technology produces an exact genetic match to proteins found on the surface of the virus, which are then expressed using Sanofi’s insect (baculovirus) expression platform. The technology is used for Sanofi’s licensed recombinant influenza vaccine and a SARS vaccine.
- Funding of up to $2.1 billion from the U.S. government as part of Operation Warp Speed.
- Supported by CEPI and Gates Foundation, funded by BARDA; agreement with TranslateBio since 2018 to deliver mRNA vax for up to five infectious diseases; busy building manufacturing facilities; Sanofi Pasteur has four genvax ‘in discovery’, meaning they have not yet begun trials; TranslateBio has four vax that can be inhaled.
- The baculovirus expression platform is protein engineering in a bioreactor, i.e. producing genetically modified proteins in insect cells.
- This platform is used to make Cervarix® and Flublok®.
Curevac
- RNActive platform with mRNA and lipid nanoparticles (LNPs); backed by Gates Foundation; partnered with CEPI; has “an extensive in-house nucleotide sequence library” and has developed a portable RNA printer; product pipeline includes CV7202 rabies vaccine, several others in pre-clinical development including cancer vax and CAS-9 gene editing.
- Their leading program, for prostate cancer vax CV0104, encoded six antigens, and is detailed in this research paper from 2014. It failed a phase II trial in 2017 and appears to have been discontinued. The only one being trialled now is CV7202 (phase I). Trials for ronavax have not yet begun.
J&J/Janssen
- Based on its AdVac and PER.C6 platform technologies; funding from BARDA;
- manufacturing capacity is being increased;
- The AdVac platform has been used in a number of clinical trials. According to this WHO document from 2016, vaccines for meningitis, RSV, ebola, prostate cancer, and Staph. Aureus had reached phase I trial, whilst vaccines for malaria, TB, HCV, HIV, and pandemic flu, had reached phase II trials.
Moderna
- mRNA1273; Moderna’s mRNA is delivered in lipid nanoparticles, description of its platform also serves to describe mRNA platforms by other companies: “… an entirely new in vivo drug technology that produces human proteins, antibodies and entirely novel protein constructs inside patient cells, which are in turn secreted or active intracellularly. This breakthrough platform addresses currently undruggable targets”
- funding from ? partnered with Merck and AstraZeneca in ? https://ir.serestherapeutics.com/news-releases/news-release-details/four-boston-biotech-firms-worth-watching
- delivered the first “clinical-grade batch” of mRNA-1273 just 42 days after sequence selection
- “Why are we so passionate about messenger RNA?” Moderna President Stephen Hoge asked the attentive audience. “It starts with the question of life,” he explained. “And in fact, all life that we know flows through messenger RNA. … In our language, mRNA is the software of life.”
- Lots of investment has been raised since it was founded in 2012 and included partnerships with DARPA, AstraZeneca and Merck and funding from the Gates Foundation.
- Generally perceived to have been operating “in stealth mode” until its plans were revealed at the J.P. Morgan Healthcare Conference in 2017.
- Moderna was the first company to publish results of a phase I study for the alleged rona, on the 18th of May, 2020. The study was conducted by the NIH.
- The results announced by the company have received criticism, plus three people experienced systemic symptoms after receiving a second injection of the high dose.
- Funding for mRNA-1273 from BARDA; manufacturing contracts with Lonza and Catalent.
- A full round up of Moderna’s product pipeline is in their SEC report, and there are tons of updates here. Their ventures include Onkaido (oncology), Valera (infectious diseases), Elpidera, (rare diseases), and Caperna, (personalized cancer vaccines).
- Moderna is using Amazon Cloud Services to help deliver personalized cancer vax.
- The phase III trial of mRNA-1273 is due to be completed in October 2022.
- Moderna has done a $1.525 billion deal to supply the US government with 100 million doses.
- The sobering reality that nobody likes to talk about is the legal bit at the end of some of the press releases: “The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna’s control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties, and other factors include, among others: the fact that there has never been a commercial product utilizing mRNA technology approved for use; the fact that the rapid response technology in use by Moderna is still being developed and implemented; the fact that the safety and efficacy of mRNA-1273 has not yet been established; potential adverse impacts due to the global COVID-19 pandemic such as delays in regulatory review, manufacturing and clinical trials, supply chain interruptions, adverse effects on healthcare systems and disruption of the global economy; and those other risks and uncertainties described under the heading “Risk Factors” in Moderna’s most recent Quarterly Report on Form 10-Q filed with the U.S. Securities and Exchange Commission (SEC) and in subsequent filings made by Moderna with the SEC, which are available on the SEC’s website at www.sec.gov”
INOVIO
- INO-4800; DNA plasmids are injected, then electrical pulses are administered to the injection site. This is called electroporation, and it’s to help the DNA get into the cells – mRNA vaccines only need to enter the cell, while DNA vaccines have to reach the cell nucleus.
- Funding from the US Dept of Defense to develop the proprietary CELLECTRA 2000 device, used for electroporation; as well as funding from Gates Foundation and CEPI.
- “To develop a new vaccine, Inovio first converts the virus’ RNA into DNA and identifies short sections that will, according to computer simulations, generate the greatest immune response. The plasmids are then produced in large quantities using bacteria”.
- The first company, partnered with GeneOne, with a MERS vaccine (GLS-5300) to get to phase II in clinical trials; the initial phase I study was conducted in partnership with the Walter Reed Army Institute of Research, and the results presented to the WHO-IVI Joint Symposium for MERS-CoV Vaccine Development in 2018.
- Development of the MERS vax was supported by a $34 million grant from the Samsung Foundation through the International Vaccine Institute, and $56 million from CEPI.
- Inovio’s pipeline includes INO-5151 for prostate cancer, INO-1800 for hepatitis B virus, GLS-6150 for hepatitis C virus, INO-4212 for Ebola virus, GLS-5300 for Middle East respiratory syndrome, GLS-5700 for Zika virus infection, PENNVAX-GP for human immunodeficiency virus and INO-4500 for Lassa fever virus.
- Development of a prophylactic vaccine has been hampered by the lack of MERS-CoV infections, “as well as sourcing suitable small animal models [75, 97, 104]. These factors complicate the definition of a target population for mass prophylactic vaccination and pre-clinical demonstration of vaccine efficacy.”
- Like the other genvax, INOVIO’s DNA plasmid vax claim to be “off-the-shelf” products, i.e. cold storage is not required, as well as rapid development times, plus they’re relatively cheap to manufacture, and large quantities can be produced.
- Partners include ApolloBio, Astra Zeneca, DARPA, National Cancer Institute, Regeneron, CEPI, Gates Foundation, and a host of others.
- INOVIO’s lead candidate is said to be VGX-3100 (for precancerous cervical dysplasia).
- INOVIO uses HEK-293t cells (this is a fetal cell line).
- The company are currently faced with lack of manufacturing facilities, but they have some strategic partnerships and plenty of products in the pipeline.
Dr Rashid Buttar: COVID-19 cepivo ima snov, ki preprečuje zanositev (sterilizacija) https://www.youtube.com/watch?v=KimrGEWqID0
* * *
Some of the Covid-19 vaccines currently in development could increase the risk of acquiring HIV, warned a group of researchers in the The Lancet medical journal Monday, potentially leading to an increase in infections as vaccines are rolled out to vulnerable populations around the world.
KEY FACTS
The researchers warn of a “cautionary tale” from efforts to create an HIV vaccine over a decade ago, where a promising vaccine candidate actually increased the risk of some men catching the virus.
The vaccine made use of a modified virus — called adenovirus 5 (Ad5) — as a vector to transport some of HIV’s genetic material into the body.
Exactly how the vaccine increased the risks of HIV transmission is unknown, but a conference convened by the National Institutes of Health recommended against further use of Ad5 as a vector in HIV vaccines (Dr. Anthony Fauci was lead author of the paper outlining this position.)
Ad5 is used as a vector in some Covid-19 vaccines — Science identifies four such candidates that are currently undergoing clinical trials in various countries around the world, including the U.S., with two in large scale phase 3 trials ongoing in Russia and Pakistan.
The researchers stressed the need to understand the role Ad5 might play in increasing the risks of HIV in vulnerable populations before developing and deploying vaccines using the vector, adding that informed consent documents should reflect the “considerable literature” on the risk of HIV acquisition with Ad5 vectors.
KEY BACKGROUND
Lots of vaccines make use of modified viruses to transport material into the human body. Many make use of a modified adenovirus to do this, a virus which is usually harmless except the ability to cause the common cold. Some of the leading candidates for a Covid-19 vaccine, including those from Johnson & Johnson and AstraZeneca, use adenoviruses as vectors. There is no evidence that those vectors increase the risk of HIV infection.
CRUCIAL QUOTE
The authors said they went public because Ad5 vaccines for Covid-19 might soon be tested in populations with high HIV prevalence. Lawrence Corey, one of the authors who now co-leads the Covid-19 prevention network in the U.S. that is testing vaccines on behalf of the NIH, told Science that if he were in a sub-Saharan African country, where there’s a high prevalence of HIV, “I don’t see why I would pick an Ad5 vector (vaccine) when there are many other alternative choices.”
FURTHER READING
Could certain COVID-19 vaccines leave people more vulnerable to the AIDS virus? (Science)
Use of adenovirus type-5 vectored vaccines: a cautionary tale (The Lancet)
Full coverage and live updates on the Coronavirus
https://www.forbes.com/sites/roberthart/2020/10/20/researchers-warn-some-covid-19-vaccines-could-increase-risk-of-hiv-infection/?fbclid=IwAR0uRjvtSN9MgYcQ03CXWwJoqosSH9qQTM0oIB8eCib2n_1vmQWLhFuwFJc#796681103740
*
https://insajder.com/svet/umrl-zdravnik-prostovoljec-ki-je-sodeloval-v-programu-preizkusa-cepiva-proti-koronavirusu?fbclid=IwAR0ywAOBvOQxiWAy51T8uitSJaNWeg4VhUxXbhEkhlngoYeqyO_vt1Ze0E4
*
https://www.rtvbn.com/3994087/nestorovic-necu-se-vakcionisati?fbclid=IwAR0IlicJVFYV-CNuYZWm-QZRocvBhHot0spFwBC_sX94tYPPNb7ijKkVLvQ Nestorovič je bivši vodja kriznega štaba za COVID-19 ukrepe v Srbiji
*
https://childrenshealthdefense.org/defender/fda-pfizer-experimental-covid-vaccine-children/?fbclid=IwAR3JtgvuQoku1YUpAY2-dxcufUpYhIIu64yeP5K7OiuYwj4S8ekMpL_ivrc
*
Hapy Yack: Publishwall 26.10. 2020: Ruski državni znanstveni center za virologijo in biotehnologijo "Vektor" pripravlja cepivo, ki bo hkrati zaščitilo ljudi pred koronavirusom SARS-CoV-2 in gripo. "Trenutno delamo na projektu enotnega cepiva proti koronavirusu in sezonski gripi," je dejal direktor te ustanove Rinat Maksjutov na zasedanju debatnega kluba "Valdaj" 21. oktobra, poroča RIA Novosti.
Kombinacija gripe in koronavirusa je zelo nevarna. Pred kratkim so znanstveniki z Liverpoolske univerze ugotovili, da če človek najprej zboli za eno, nato pa za drugo boleznijo, je smrtno tveganje šestkrat večje. Trenutno v Rusiji poteka intenzivno cepljenje proti gripi.
Moskovski župan Sergej Sobjanin je pred kratkim napovedal, da se bo množično cepljenje proti novemu koronavirusu v ruski prestolnici začelo decembra oz. januarja.
*
Cepivo Moderne za COVID-19 ne preprečuje prenosa okužbe, bolezni niti smrti. Uustavi samo blage simptome, kot je kašelj in ne same bolezni : (večina ljudi simptomov sploh nima):
https://www.bmj.com/content/371/bmj.m4037
Dr Anthony Fauci quoted by Megan Sheets, Daily Mail [1].
So, what might happen if people have suppressed symptoms but are contagious? And what will doctors tell their patients if vaccines are rolled out as early as next month [2,3].
[1] Megan Sheets, ‘ Dr Fauci warns that early COVID-19 vaccines will only prevent symptoms from arising - not block infection’, Daily Mail 27 October 2020, https://www.dailymail.co.uk/news/article-8884031/Dr-Fauci-warns-early-CO...
https://www.bmj.com/content/371/bmj.m4037/rapid-responses
*
https://www.chemistryworld.com/news/what-are-the-risks-of-fast-tracking-a-covid-19-vaccine/4012130.article
*
Dr Peter Hotez : okrepeljen imunski odziv cepiva za SARS in umiranje cepljenih živali (min 1.25.00) https://thehighwire.com/videos/the-vaccine-safety-project/?fbclid=IwAR0Z-hpK5A0rlEZmaf8yPR505JGQ3u_2Q80r_fEBXC8vnvhCUc_k9VpoiLQ
Dr. Dolores Cahill ADE in umiranje cepljenih živali https://brandnewtube.com/watch/sfK2lFUVeYySRqH&cl=149120
Študija na mucah: Various vaccines designed to prevent FIPV infection have been shown to exacerbate the disease, probably due to immune enhancement mediated by virus-specific immunoglobulins against the outer envelope (S) protein.
https://pubmed.ncbi.nlm.nih.gov/8830483/
Pljučna patologija pri miših po prejemu cepiva za koronavirus SARS 1 https://pubmed.ncbi.nlm.nih.gov/22536382/
*
FB 4.12. 2020
Jul 23, 2020